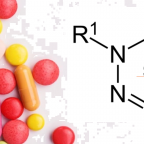
(PRESS RELEASE) GENEVA, 26-Mar-2020 — /EuropaWire/ — Inspection, verification, testing and certification company SGS announced it has developed a test method to identify nitrosamine impurities in drug products, raw materials and APIs. In 2018, the presence of the nitrosamine N-nitrosodimethylamine … Read the full press release →
Posted in Healthcare, News, Pharma & Biotech, Science, Switzerland
Tagged carcinogen, contaminated products, EMA, FDA, genotoxins, Mark Rogers, medicines, metformin, N-nitrosamine formation, NDMA, NDMA traces, nitrosamine, nitrosamine impurities, Ranitidine, regulatory warnings















