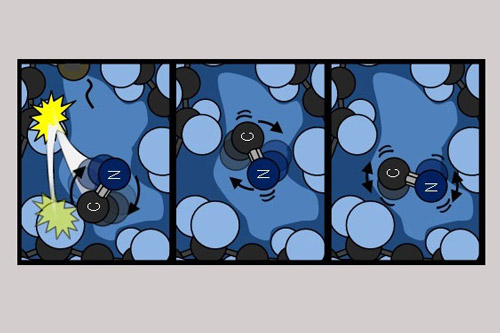
A hot CN molecule cools in solution by collisions with solvent molecules that reduce both its speed and how quickly it is rotating. The three panels show schematic snapshots of this cooling at successively later times as the CN settles down from free movement to hindered motion that is restricted by the surrounding solvent molecules. Image credit: Dr Michael Grubb (University of Bristol)
how-energy-flows-from-hot-molecule-into-surrounding-liquid-revealed-by-university-of-bristol-scientists
Published 11, October 2016 at 500 × 333 in How energy flows from hot molecule into surrounding liquid revealed by University of Bristol scientists
Bookmark the permalink.