Positive phase III results for Roche’s emicizumab in haemophilia A published in The New England Journal of Medicine
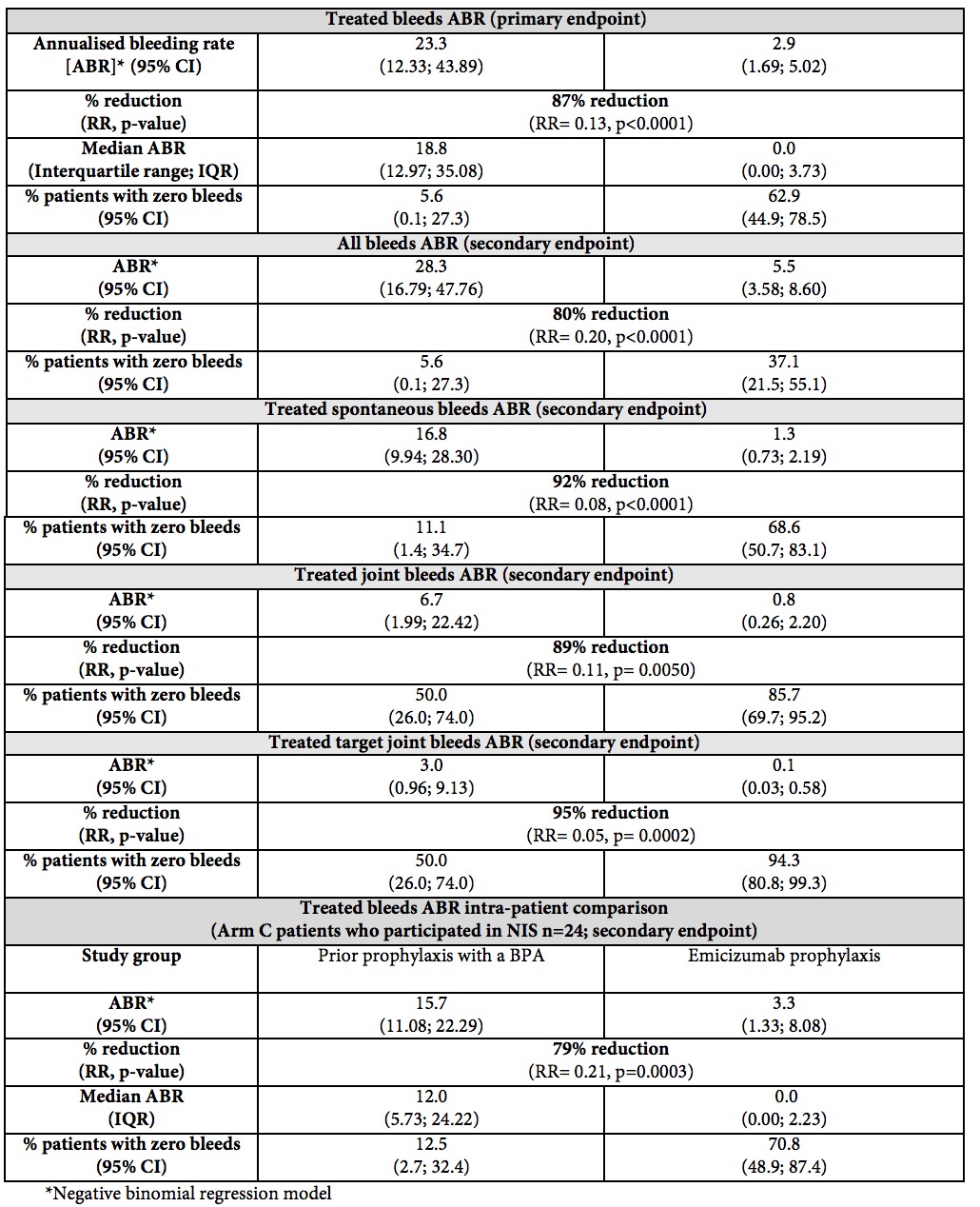
HAVEN 1 showed emicizumab reduced bleed rate by 87% compared with on-demand bypassing agents
All 12 secondary endpoints in HAVEN 1 were positive, including an intra-patient comparison that showed emicizumab reduced bleed rate by 79% compared to prior prophylactic bypassing agents
Data from HAVEN 1 in adults and adolescents and interim data from HAVEN 2 in children with haemophilia A with inhibitors are being presented today at the 26th International Society on Thrombosis and Haemostasis (ISTH) Congress
Data from both studies have been submitted to FDA and EMA for approval consideration
BASEL, 11-Jul-2017 — /EuropaWire/ — Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that data from HAVEN 1, a phase III study evaluating once-weekly subcutaneous emicizumab prophylaxis (preventative) in adults and adolescents with haemophilia A with inhibitors, were published in The New England Journal of Medicine (NEJM). The primary endpoint showed a clinically meaningful and statistically significant reduction in treated bleeds of 87% (risk rate [RR]=0.13, p<0.0001) with emicizumab prophylaxis compared with on-demand (no prophylaxis; episodic use only) bypassing agents (BPAs). All 12 secondary endpoints were positive, including a statistically significant reduction of 79% (RR=0.21, p=0.0003) in treated bleeds in a first of its kind intra-patient analysis in a subset of patients comparing two prophylaxis regimens (emicizumab and BPAs). Data from HAVEN 1 as well as the interim analysis of the phase III HAVEN 2 study of emicizumab in children are being presented at the 26th International Society on Thrombosis and Haemostasis (ISTH) Congress today.
“Nearly one in three people with haemophilia A develop inhibitors to standard factor VIII therapy, leaving them at greater risk of life-threatening bleeds and long-term joint damage,” said Sandra Horning, MD, Roche’s Chief Medical Officer and Head of Global Product Development. “Based on the bleed reduction shown in the HAVEN 1 and HAVEN 2 studies, we believe emicizumab has the potential to make a meaningful difference for people with haemophilia A with inhibitors, while also reducing the burden of managing the condition with a subcutaneous, once-weekly administration.”
Further data from HAVEN 1 showed that, after a median observation time of 31 weeks, substantially more patients experienced zero bleeds with emicizumab prophylaxis than with on-demand BPAs across all bleed measurements, including zero treated bleeds (62.9% vs 5.6%), zero treated spontaneous bleeds (68.6% vs 11.1%), zero treated joint bleeds (85.7% vs 50.0%), zero treated target joint bleeds (94.3% vs 50.0%) and zero bleeds overall, which includes all treated and non-treated bleeds (37.1% vs 5.6%). A clinically meaningful and statistically significant improvement in health-related quality of life (HRQoL) measured at 25 weeks, using two validated instruments (Haem-A-QoL and EQ-5D-5L), was also observed.
In an additional study arm (Arm C, n=49), patients who had previously received BPA prophylaxis were treated with emicizumab prophylaxis. A subset of patients in this arm (n=24) had previously participated in a non-interventional study (NIS), allowing for a first of its kind intra-patient analysis comparing two prophylaxis regimens. This analysis showed a 79% (RR=0.21, p=0.0003) reduction in treated bleeds in patients receiving emicizumab compared with their prior BPA prophylaxis during the NIS. Data also showed that 70.8% of patients in this subset experienced zero treated bleeds with emicizumab prophylaxis whereas only 12.5% of these patients had experienced zero bleeds with their prior BPA prophylaxis during the NIS.
“The HAVEN 1 study is one of the most robust clinical studies conducted to date in people with haemophilia A with inhibitors to factor VIII, including a first ever intra-patient comparison to prior prophylaxis with bypassing agents,” said Professor Johannes Oldenburg, Institute of Experimental Haematology and Transfusion Medicine, University of Bonn, Germany. “The reduction in bleeding events across all measures seen with emicizumab compared to either on-demand or prophylactic bypassing agents supports that it may be one of the most significant scientific innovations in the treatment of haemophilia A in over 30 years.”
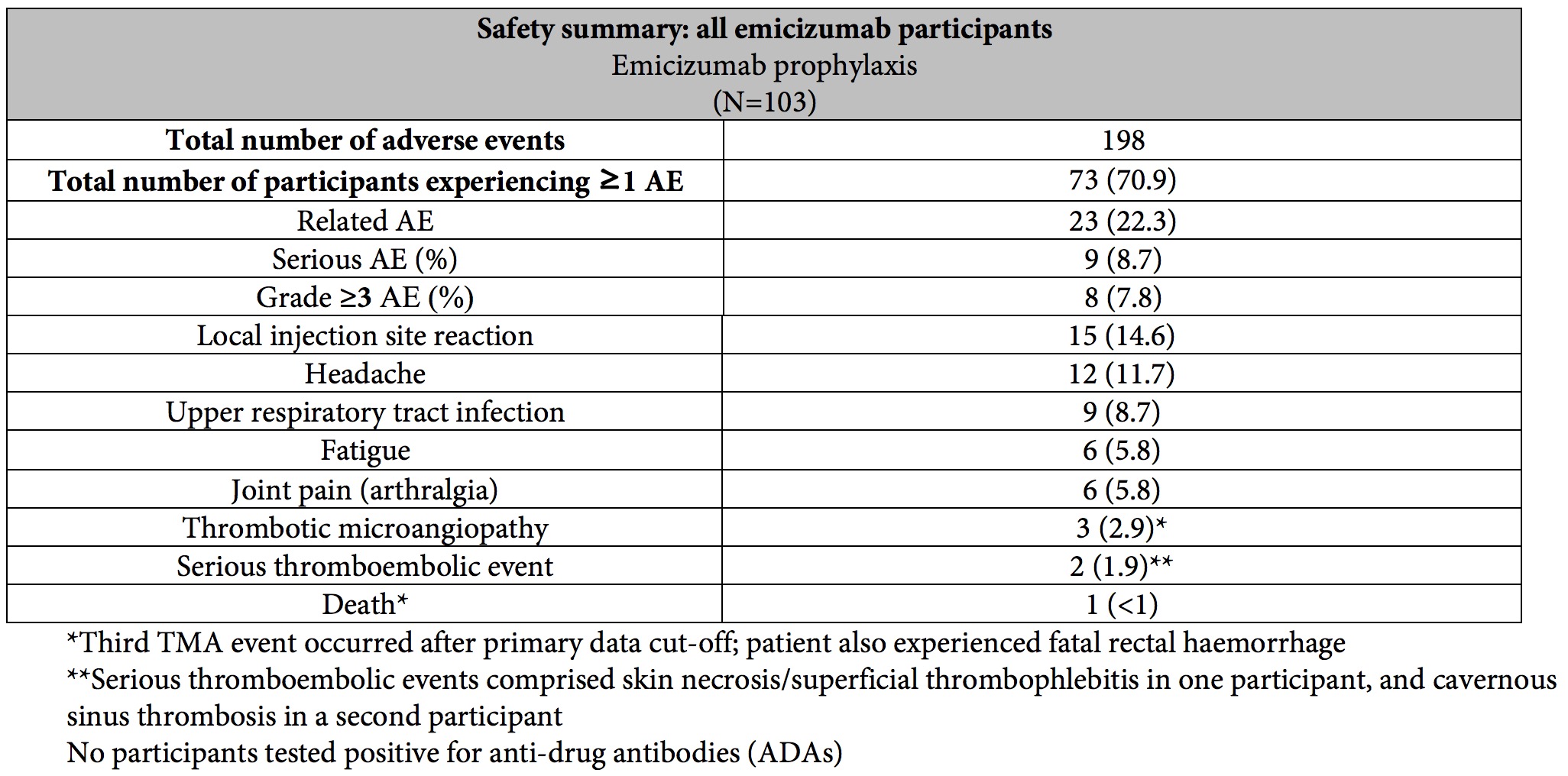
Adverse events (AEs) occurring in 5% or more of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection and joint pain (arthralgia). As previously reported, serious adverse events of thromboembolic events (TE) and thrombotic microangiopathy (TMA) occurred in two patients and three patients*, respectively, while receiving emicizumab prophylaxis. The common aspect of these TMA** and TE events is the patients were on emicizumab prophylaxis and received more than 100 u/kg/day of the BPA activated prothrombin complex on average for 24 hours or more before the onset of the event. Neither TE event required anti-coagulation therapy and one patient restarted emicizumab. The cases of TMA observed were transient, and one patient restarted emicizumab.
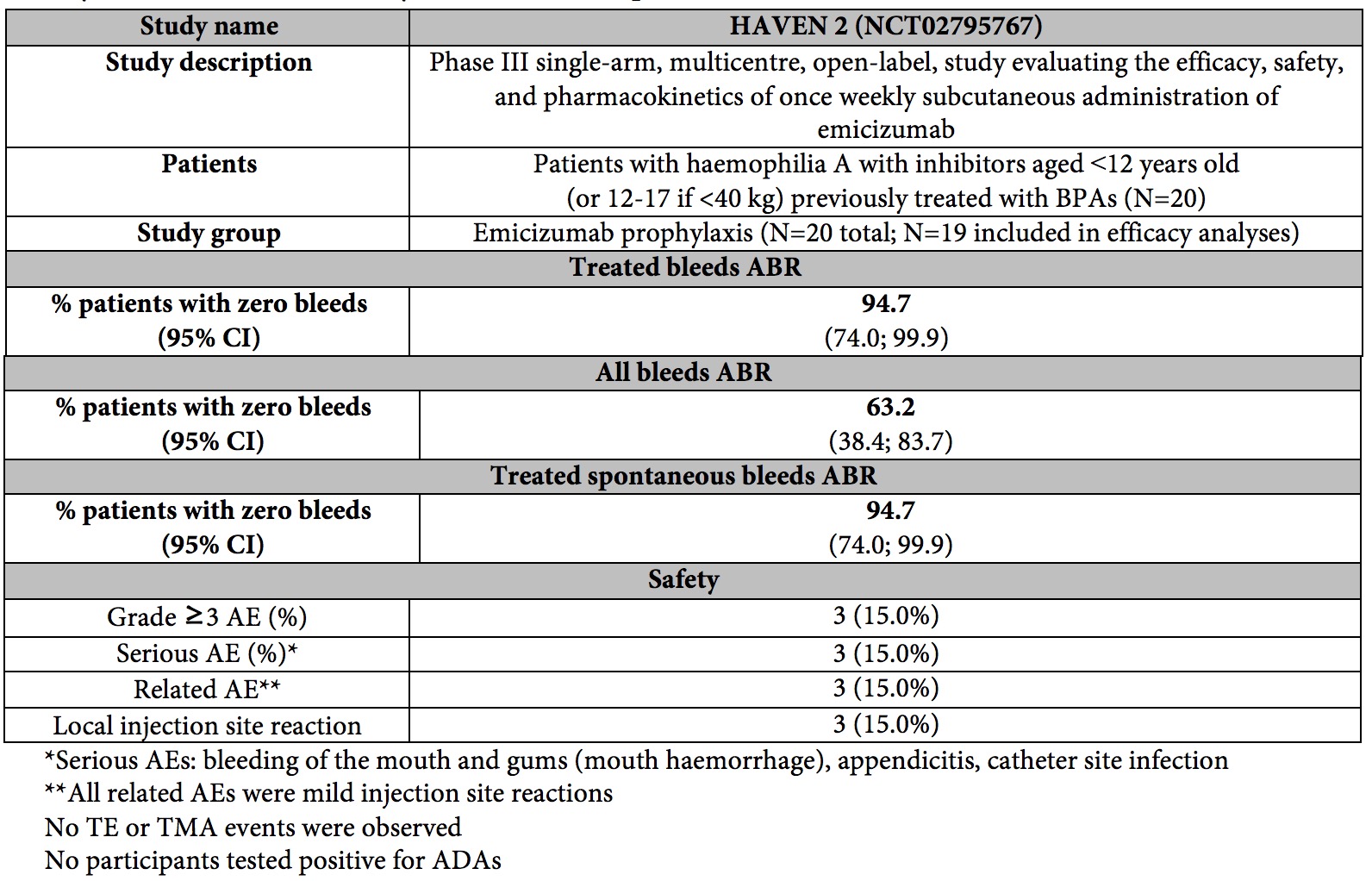
Interim results from the single arm HAVEN 2 study in children younger than 12 years of age with haemophilia A with inhibitors who received emicizumab prophylaxis are consistent with the positive results from the HAVEN 1 study. After a median observation time of 12 weeks, the study showed that only one of 19 children receiving emicizumab reported a treated bleed. There were no reported joint or muscle bleeds.
An intra-patient comparison (n=8) in a subset of these children who were previously enrolled in the NIS, showed that all experienced a 100% reduction in treated bleeds following treatment with emicizumab (previous annualised bleeding rate [ABR] ranged from 0 to 34.24); this group included seven children who had received prior BPA prophylaxis, and one who had received prior on-demand BPA. The data also indicate that the same dose of emicizumab is appropriate for children as for adults and adolescents, based on the levels of emicizumab in the blood (pharmacokinetics) of the children compared with the levels of emicizumab in the blood of adults and adolescents. The most common AEs with emicizumab in the HAVEN 2 study were mild injection site reactions and common cold symptoms (nasopharyngitis). No TE or TMA events were observed.
“Managing haemophilia A with inhibitors to factor VIII can be especially challenging for children and their caregivers. Not only can bleeding be difficult to control, but current treatments can require frequent intravenous infusions, which can often involve the long-term use of a central venous access device or port,” said Guy Young, MD, Director of Hemostasis and Thrombosis Program, Children’s Hospital Los Angeles, and Professor of Pediatrics, University of Southern California Keck School of Medicine, Los Angeles, California. “The HAVEN 2 interim results indicate that emicizumab may help prevent bleeding in children with inhibitors. Given the once-weekly subcutaneous dosing, it may also help alleviate some of the burden of haemophilia treatment for these children and their parents.”
Data from both HAVEN 1 and HAVEN 2 have been submitted for approval consideration to the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA). The FDA granted Breakthrough Therapy Designation for emicizumab in adults and adolescents with haemophilia A with inhibitors in September 2015. Additional studies evaluating emicizumab in people with haemophilia A both with and without inhibitors and exploring less frequent dosing regimens are ongoing.
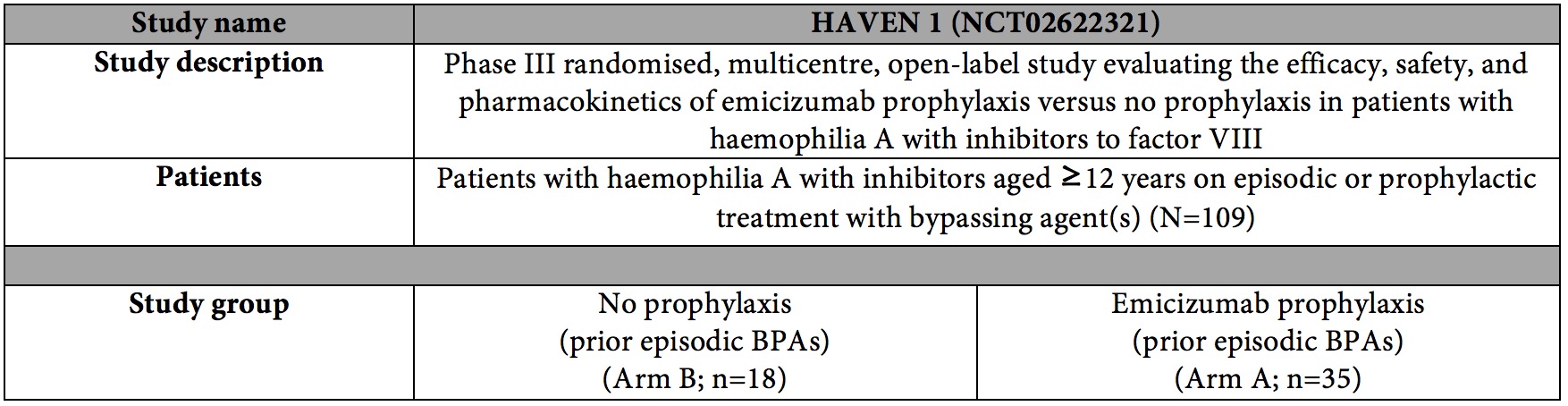
About HAVEN 1 (NCT02622321)
HAVEN 1 is a randomised, multicentre, open-label, phase III study evaluating the efficacy, safety, and pharmacokinetics of emicizumab prophylaxis compared to on-demand BPA (no prophylaxis; episodic use only) in adults and adolescents with haemophilia A with inhibitors to factor VIII. The study included 109 patients (12 years of age or older) with haemophilia A with inhibitors to factor VIII, who were previously treated with BPAs on-demand or as prophylaxis. Patients previously treated with on-demand BPAs were randomised in a 2:1 fashion to receive emicizumab prophylaxis (Arm A) or no prophylaxis (Arm B). Patients previously treated with prophylactic BPAs received emicizumab prophylaxis (Arm C). Additional patients previously on BPA (on-demand or prophylaxis) were also enrolled in a separate arm (Arm D). On-demand treatment of breakthrough bleeds with BPAs was allowed per protocol in all arms.
The primary endpoint of the study is the number of treated bleeds over time with emicizumab prophylaxis (Arm A) compared with no prophylaxis (Arm B). Secondary endpoints include all bleed rate, joint bleed rate, spontaneous bleed rate, target joint bleed rate, HRQoL/ health status, intra-patient comparison to bleed rate on their prior prophylaxis regimen with BPAs (Arm C) or no prophylaxis (Arm B). The study also evaluated safety and pharmacokinetics.
A summary of the HAVEN 1 study results to be presented at ISTH is included below.
About HAVEN 2 (NCT02795767)
HAVEN 2 is a single-arm, multicentre, open-label, phase III study evaluating the efficacy, safety, and pharmacokinetics of once-weekly subcutaneous administration of emicizumab. The interim analysis after a median of 12 weeks of treatment included 19 children younger than 12 years of age with haemophilia A with inhibitors to factor VIII, who require treatment with BPAs. The objectives of the study are to evaluate the number of treated bleeds over time with emicizumab prophylaxis, safety, pharmacokinetics, HRQoL and proxy HRQoL with aspects of caregiver burden.
A summary of the HAVEN 2 study interim results presented at ISTH is included below.
About emicizumab (ACE910)
Emicizumab is an investigational bispecific monoclonal antibody designed to bring together factors IXa and X, proteins required to activate the natural coagulation cascade and restore the blood clotting process. Emicizumab can be administered by an injection of a ready-to-use solution under the skin (subcutaneously) once weekly. Emicizumab is being evaluated in pivotal phase III studies in people 12 years of age and older, both with and without inhibitors to factor VIII, and in children under 12 years of age with factor VIII inhibitors. Additional trials are exploring less frequent dosing schedules. The clinical development programme is assessing the safety and efficacy of emicizumab and its potential to help overcome current clinical challenges: the short-lasting effects of existing treatments, the development of factor VIII inhibitors and the need for frequent venous access. Emicizumab was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche and Genentech.
About haemophilia A
Haemophilia A is an inherited, serious disorder in which a person’s blood does not clot properly, leading to uncontrolled and often spontaneous bleeding. Haemophilia A affects around 320,000 people worldwide1,2 approximately 50-60% of whom have a severe form of the disorder.3 People with haemophilia A either lack or do not have enough of a clotting protein called factor VIII. In a healthy person, when a bleed occurs, factor VIII brings together the clotting factors IXa and X, which is a critical step in the formation of a blood clot to help stop bleeding. Depending on the severity of their disorder, people with haemophilia A can bleed frequently, especially into their joints or muscles.1 These bleeds can present a significant health concern as they often cause pain and can lead to chronic swelling, deformity, reduced mobility, and long-term joint damage.4 In addition to impacting a person’s quality of life,5 these bleeds can be life threatening if they go into vital organs, such as the brain.6,7
A serious complication of treatment is the development of inhibitors to factor VIII replacement therapies.8 Inhibitors are antibodies developed by the body’s immune system that bind to and block the efficacy of replacement factor VIII,9 making it difficult, if not impossible to obtain a level of factor VIII sufficient to control bleeding. Most people with haemophilia A who develop inhibitors will infuse BPA therapies, either on-demand (episodic) or as prophylaxis, to control bleeding. This approach is known to be less effective and less predictable than factor VIII replacement therapy in people with haemophilia A without inhibitors.10
About Roche in haematology
For more than 20 years, Roche has been developing medicines that redefine treatment in haematology. Today, we are investing more than ever in our effort to bring innovative treatment options to people with diseases of the blood. In addition to approved medicines MabThera®/Rituxan® (rituximab), Gazyva®/Gazyvaro® (obinutuzumab), and Venclexta™/Venclyxto™ (venetoclax) in collaboration with AbbVie, Roche’s pipeline of investigational haematology medicines includes Tecentriq® (atezolizumab), an anti-CD79b antibody drug conjugate (polatuzumab vedotin/RG7596) and a small molecule antagonist of MDM2 (idasanutlin/RG7388). Roche’s dedication to developing novel molecules in haematology expands beyond malignancy, with the development of the investigational haemophilia A treatment emicizumab (ACE910).
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. Thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Roche has been recognised as the Group Leader in sustainability within the Pharmaceuticals, Biotechnology & Life Sciences Industry eight years in a row by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2016 employed more than 94,000 people worldwide. In 2016, Roche invested CHF 9.9 billion in R&D and posted sales of CHF 50.6 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com.
All trademarks used or mentioned in this release are protected by law.
References
*One event occurred after the clinical cut-off date for the primary analysis
** Two of these patients had also received recombinant FVIIa (rFVII).
WFH. Guidelines for the management of hemophilia. 2012. Last accessed July 2017: http://www1.wfh.org/publications/files/pdf-1472.pdf
Berntorp E, Shapiro AD. Modern haemophilia care. The Lancet 2012; 370:1447-1456.
Marder VJ, et al. Hemostasis and Thrombosis. Basic Principles and Clinical Practice. 6th Edition, 2013. Milwakee, Wisconsin. Lippincott Williams and Wilkin.
Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood Rev 2013; 179-84.
Flood, E et al. Illustrating the impact of mild/moderate and severe haemophilia on health-related quality of life: hypothesised conceptual models. European Journal of Haematology 2014; 93: Suppl. 75, 9–18.
Young G. New challenges in hemophilia: long-term outcomes and complications. Hematology Am Soc Hematol Educ Program 2012. 2012; 362–8.
Zanon E, Iorio A, Rocino A, et al. Intracranial haemorrhage in the Italian population of haemophilia patients with and without inhibitors. Haemophilia 2012; 18: 39–45.
Gomez K, et al. Key issues in inhibitor management in patients with haemophilia. Blood Transfus. 2014; 12:s319–s329.
Whelan, SF, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood 2013; 121: 1039-48
Berntorp E. Differential response to bypassing agents complicates treatment in patients with haemophilia and inhibitors. Haemophilia 2009; 15:3-10.
SOURCE: F. Hoffmann-La Roche Ltd
Media contacts
Email Media Team
+41 61 688 8888
- BREAKING NEWS: New Podcast “Spreading the Good BUZZ” Hosted by Josh and Heidi Case Launches July 7th with Explosive Global Reach and a Mission to Transform Lives Through Hope and Community in Recovery
- Cha Cha Cha kohtub krüptomaailmaga: Winz.io teeb koostööd Euroopa visionääri ja staari Käärijäga
- Digi Communications N.V. announces Conditional stock options granted to Executive Directors of the Company, for the year 2025, based on the general shareholders’ meeting approval from 25 June 20244
- Cha Cha Cha meets crypto: Winz.io partners with European visionary star Käärijä
- Digi Communications N.V. announces the exercise of conditional share options by the executive directors of the Company, for the year 2024, as approved by the Company’s OGSM from 25 June 2024
- “Su Fortuna Se Ha Construido A Base de La Defraudación Fiscal”: Críticas Resurgen Contra Ricardo Salinas en Medio de Nuevas Revelaciones Judiciales y Fiscaleso
- Digi Communications N.V. announces the availability of the instruction regarding the payment of share dividend for the 2024 financial year
- SOILRES project launches to revive Europe’s soils and future-proof farming
- Josh Case, ancien cadre d’ENGIE Amérique du Nord, PDG de Photosol US Renewable Energy et consultant d’EDF Amérique du Nord, engage aujourd’hui toute son énergie dans la lutte contre la dépendance
- Bizzy startet den AI Sales Agent in Deutschland: ein intelligenter Agent zur Automatisierung der Vertriebspipeline
- Bizzy lance son agent commercial en France : un assistant intelligent qui automatise la prospection
- Bizzy lancia l’AI Sales Agent in Italia: un agente intelligente che automatizza la pipeline di vendita
- Bizzy lanceert AI Sales Agent in Nederland: slimme assistent automatiseert de sales pipeline
- Bizzy startet AI Sales Agent in Österreich: ein smarter Agent, der die Sales-Pipeline automatisiert
- Bizzy wprowadza AI Sales Agent w Polsce: inteligentny agent, który automatyzuje budowę lejka sprzedaży
- Bizzy lanza su AI Sales Agent en España: un agente inteligente que automatiza la generación del pipeline de ventas
- Bizzy launches AI Sales Agent in the UK: a smart assistant that automates sales pipeline generation
- As Sober.Buzz Community Explodes Its Growth Globally it is Announcing “Spreading the Good BUZZ” Podcast Hosted by Josh Case Debuting July 7th
- Digi Communications N.V. announces the OGMS resolutions and the availability of the approved 2024 Annual Report
- Escándalo Judicial en Aumento Alarma a la Opinión Pública: Suprema Corte de México Enfrenta Acusaciones de Favoritismo hacia el Aspirante a Magnate Ricardo Salinas Pliego
- Winz.io Named AskGamblers’ Best Casino 2025
- Kissflow Doubles Down on Germany as a Strategic Growth Market with New AI Features and Enterprise Focus
- Digi Communications N.V. announces Share transaction made by a Non-Executive Director of the Company with class B shares
- Salinas Pliego Intenta Frenar Investigaciones Financieras: UIF y Expertos en Corrupción Prenden Alarmas
- Digital integrity at risk: EU Initiative to strengthen the Right to be forgotten gains momentum
- Orden Propuesta De Arresto E Incautación Contra Ricardo Salinas En Corte De EE.UU
- Digi Communications N.V. announced that Serghei Bulgac, CEO and Executive Director, sold 15,000 class B shares of the company’s stock
- PFMcrypto lancia un sistema di ottimizzazione del reddito basato sull’intelligenza artificiale: il mining di Bitcoin non è mai stato così facile
- Azteca Comunicaciones en Quiebra en Colombia: ¿Un Presagio para Banco Azteca?
- OptiSigns anuncia su expansión Europea
- OptiSigns annonce son expansion européenne
- OptiSigns kündigt europäische Expansion an
- OptiSigns Announces European Expansion
- Digi Communications NV announces release of Q1 2025 financial report
- Banco Azteca y Ricardo Salinas Pliego: Nuevas Revelaciones Aumentan la Preocupación por Riesgos Legales y Financieros
- Digi Communications NV announces Investors Call for the presentation of the Q1 2025 Financial Results
- Digi Communications N.V. announces the publication of the 2024 Annual Financial Report and convocation of the Company’s general shareholders meeting for June 18, 2025, for the approval of, among others, the 2024 Annual Financial Report, available on the Company’s website
- La Suprema Corte Sanciona a Ricardo Salinas de Grupo Elektra por Obstrucción Legal
- Digi Communications N.V. announces the conclusion of an Incremental to the Senior Facilities Agreement dated 21 April 2023
- 5P Europe Foundation: New Initiative for African Children
- 28-Mar-2025: Digi Communications N.V. announces the conclusion of Facilities Agreements by companies within Digi Group
- Aeroluxe Expeditions Enters U.S. Market with High-Touch Private Jet Journeys—At a More Accessible Price ↗️
- SABIO GROUP TAKES IT’S ‘DISRUPT’ CX PROGRAMME ACROSS EUROPE
- EU must invest in high-quality journalism and fact-checking tools to stop disinformation
- ¿Está Banco Azteca al borde de la quiebra o de una intervención gubernamental? Preocupaciones crecientes sobre la inestabilidad financiera
- Netmore and Zenze Partner to Deploy LoRaWAN® Networks for Cargo and Asset Monitoring at Ports and Terminals Worldwide
- Rise Point Capital: Co-investing with Independent Sponsors to Unlock International Investment Opportunities
- Netmore Launches Metering-as-a-Service to Accelerate Smart Metering for Water and Gas Utilities
- Digi Communications N.V. announces that a share transaction was made by a Non-Executive Director of the Company with class B shares
- La Ballata del Trasimeno: Il Mediometraggio si Trasforma in Mini Serie
- Digi Communications NV Announces Availability of 2024 Preliminary Financial Report
- Digi Communications N.V. announces the recent evolution and performance of the Company’s subsidiary in Spain
- BevZero Equipment Sales and Distribution Enhances Dealcoholization Capabilities with New ClearAlc 300 l/h Demonstration Unit in Spain Facility
- Digi Communications NV announces Investors Call for the presentation of the 2024 Preliminary Financial Results
- Reuters webinar: Omnibus regulation Reuters post-analysis
- Patients as Partners® Europe Launches the 9th Annual Event with 2025 Keynotes, Featured Speakers and Topics
- eVTOLUTION: Pioneering the Future of Urban Air Mobility
- Reuters webinar: Effective Sustainability Data Governance
- Las acusaciones de fraude contra Ricardo Salinas no son nuevas: una perspectiva histórica sobre los problemas legales del multimillonario
- Digi Communications N.V. Announces the release of the Financial Calendar for 2025
- USA Court Lambasts Ricardo Salinas Pliego For Contempt Of Court Order
- 3D Electronics: A New Frontier of Product Differentiation, Thinks IDTechEx
- Ringier Axel Springer Polska Faces Lawsuit for Over PLN 54 million
- Digi Communications N.V. announces the availability of the report on corporate income tax information for the financial year ending December 31, 2023
- Unlocking the Multi-Million-Dollar Opportunities in Quantum Computing
- Digi Communications N.V. Announces the Conclusion of Facilities Agreements by Companies within Digi Group
- The Hidden Gem of Deep Plane Facelifts
- KAZANU: Redefining Naturist Hospitality in Saint Martin ↗️
- New IDTechEx Report Predicts Regulatory Shifts Will Transform the Electric Light Commercial Vehicle Market
- Almost 1 in 4 Planes Sold in 2045 to be Battery Electric, Finds IDTechEx Sustainable Aviation Market Report
- Digi Communications N.V. announces the release of Q3 2024 financial results
- Digi Communications NV announces Investors Call for the presentation of the Q3 2024 Financial Results
- Pilot and Electriq Global announce collaboration to explore deployment of proprietary hydrogen transport, storage and power generation technology
- Digi Communications N.V. announces the conclusion of a Memorandum of Understanding by its subsidiary in Romania
- Digi Communications N.V. announces that the Company’s Portuguese subsidiary finalised the transaction with LORCA JVCO Limited
- Digi Communications N.V. announces that the Portuguese Competition Authority has granted clearance for the share purchase agreement concluded by the Company’s subsidiary in Portugal
- OMRON Healthcare introduceert nieuwe bloeddrukmeters met AI-aangedreven AFib-detectietechnologie; lancering in Europa september 2024
- OMRON Healthcare dévoile de nouveaux tensiomètres dotés d’une technologie de détection de la fibrillation auriculaire alimentée par l’IA, lancés en Europe en septembre 2024
- OMRON Healthcare presenta i nuovi misuratori della pressione sanguigna con tecnologia di rilevamento della fibrillazione atriale (AFib) basata sull’IA, in arrivo in Europa a settembre 2024
- OMRON Healthcare presenta los nuevos tensiómetros con tecnología de detección de fibrilación auricular (FA) e inteligencia artificial (IA), que se lanzarán en Europa en septiembre de 2024
- Alegerile din Moldova din 2024: O Bătălie pentru Democrație Împotriva Dezinformării
- Northcrest Developments launches design competition to reimagine 2-km former airport Runway into a vibrant pedestrianized corridor, shaping a new era of placemaking on an international scale
- The Road to Sustainable Electric Motors for EVs: IDTechEx Analyzes Key Factors
- Infrared Technology Breakthroughs Paving the Way for a US$500 Million Market, Says IDTechEx Report
- MegaFair Revolutionizes the iGaming Industry with Skill-Based Games
- European Commission Evaluates Poland’s Media Adherence to the Right to be Forgotten
- Global Race for Autonomous Trucks: Europe a Critical Region Transport Transformation
- Digi Communications N.V. confirms the full redemption of €450,000,000 Senior Secured Notes
- AT&T Obtiene Sentencia Contra Grupo Salinas Telecom, Propiedad de Ricardo Salinas, Sus Abogados se Retiran Mientras Él Mueve Activos Fuera de EE.UU. para Evitar Pagar la Sentencia
- Global Outlook for the Challenging Autonomous Bus and Roboshuttle Markets
- Evolving Brain-Computer Interface Market More Than Just Elon Musk’s Neuralink, Reports IDTechEx
- Latin Trails Wraps Up a Successful 3rd Quarter with Prestigious LATA Sustainability Award and Expands Conservation Initiatives ↗️
- Astor Asset Management 3 Ltd leitet Untersuchung für potenzielle Sammelklage gegen Ricardo Benjamín Salinas Pliego von Grupo ELEKTRA wegen Marktmanipulation und Wertpapierbetrug ein
- Digi Communications N.V. announces that the Company’s Romanian subsidiary exercised its right to redeem the Senior Secured Notes due in 2025 in principal amount of €450,000,000
- Astor Asset Management 3 Ltd Inicia Investigación de Demanda Colectiva Contra Ricardo Benjamín Salinas Pliego de Grupo ELEKTRA por Manipulación de Acciones y Fraude en Valores
- Astor Asset Management 3 Ltd Initiating Class Action Lawsuit Inquiry Against Ricardo Benjamín Salinas Pliego of Grupo ELEKTRA for Stock Manipulation & Securities Fraud
- Digi Communications N.V. announced that its Spanish subsidiary, Digi Spain Telecom S.L.U., has completed the first stage of selling a Fibre-to-the-Home (FTTH) network in 12 Spanish provinces
- Natural Cotton Color lancia la collezione "Calunga" a Milano
- Astor Asset Management 3 Ltd: Salinas Pliego Incumple Préstamo de $110 Millones USD y Viola Regulaciones Mexicanas
- Astor Asset Management 3 Ltd: Salinas Pliego Verstößt gegen Darlehensvertrag über 110 Mio. USD und Mexikanische Wertpapiergesetze
- ChargeEuropa zamyka rundę finansowania, której przewodził fundusz Shift4Good tym samym dokonując historycznej francuskiej inwestycji w polski sektor elektromobilności
- Strengthening EU Protections: Robert Szustkowski calls for safeguarding EU citizens’ rights to dignity
- Digi Communications NV announces the release of H1 2024 Financial Results
- Digi Communications N.V. announces that conditional stock options were granted to a director of the Company’s Romanian Subsidiary
- Digi Communications N.V. announces Investors Call for the presentation of the H1 2024 Financial Results
- Digi Communications N.V. announces the conclusion of a share purchase agreement by its subsidiary in Portugal
- Digi Communications N.V. Announces Rating Assigned by Fitch Ratings to Digi Communications N.V.
- Digi Communications N.V. announces significant agreements concluded by the Company’s subsidiaries in Spain
- SGW Global Appoints Telcomdis as the Official European Distributor for Motorola Nursery and Motorola Sound Products
- Digi Communications N.V. announces the availability of the instruction regarding the payment of share dividend for the 2023 financial year
- Digi Communications N.V. announces the exercise of conditional share options by the executive directors of the Company, for the year 2023, as approved by the Company’s Ordinary General Shareholders’ Meetings from 18th May 2021 and 28th December 2022
- Digi Communications N.V. announces the granting of conditional stock options to Executive Directors of the Company based on the general shareholders’ meeting approval from 25 June 2024
- Digi Communications N.V. announces the OGMS resolutions and the availability of the approved 2023 Annual Report
- Czech Composer Tatiana Mikova Presents Her String Quartet ‘In Modo Lidico’ at Carnegie Hall
- SWIFTT: A Copernicus-based forest management tool to map, mitigate, and prevent the main threats to EU forests
- WickedBet Unveils Exciting Euro 2024 Promotion with Boosted Odds
- Museum of Unrest: a new space for activism, art and design
- Digi Communications N.V. announces the conclusion of a Senior Facility Agreement by companies within Digi Group
- Digi Communications N.V. announces the agreements concluded by Digi Romania (formerly named RCS & RDS S.A.), the Romanian subsidiary of the Company
- Green Light for Henri Hotel, Restaurants and Shops in the “Alter Fischereihafen” (Old Fishing Port) in Cuxhaven, opening Summer 2026
- Digi Communications N.V. reports consolidated revenues and other income of EUR 447 million, adjusted EBITDA (excluding IFRS 16) of EUR 140 million for Q1 2024
- Digi Communications announces the conclusion of Facilities Agreements by companies from Digi Group
- Digi Communications N.V. Announces the convocation of the Company’s general shareholders meeting for 25 June 2024 for the approval of, among others, the 2023 Annual Report
- Digi Communications NV announces Investors Call for the presentation of the Q1 2024 Financial Results
- Digi Communications intends to propose to shareholders the distribution of dividends for the fiscal year 2023 at the upcoming General Meeting of Shareholders, which shall take place in June 2024
- Digi Communications N.V. announces the availability of the Romanian version of the 2023 Annual Report
- Digi Communications N.V. announces the availability of the 2023 Annual Report
- International Airlines Group adopts Airline Economics by Skailark ↗️
- BevZero Spain Enhances Sustainability Efforts with Installation of Solar Panels at Production Facility
- Digi Communications N.V. announces share transaction made by an Executive Director of the Company with class B shares
- BevZero South Africa Achieves FSSC 22000 Food Safety Certification
- Digi Communications N.V.: Digi Spain Enters Agreement to Sell FTTH Network to International Investors for Up to EUR 750 Million
- Patients as Partners® Europe Announces the Launch of 8th Annual Meeting with 2024 Keynotes and Topics
- driveMybox continues its international expansion: Hungary as a new strategic location
- Monesave introduces Socialised budgeting: Meet the app quietly revolutionising how users budget
- Digi Communications NV announces the release of the 2023 Preliminary Financial Results
- Digi Communications NV announces Investors Call for the presentation of the 2023 Preliminary Financial Results
- Lensa, един от най-ценените търговци на оптика в Румъния, пристига в България. Първият шоурум е открит в София
- Criando o futuro: desenvolvimento da AENO no mercado de consumo em Portugal
- Digi Communications N.V. Announces the release of the Financial Calendar for 2024
- Customer Data Platform Industry Attracts New Participants: CDP Institute Report
- eCarsTrade annonce Dirk Van Roost au poste de Directeur Administratif et Financier: une décision stratégique pour la croissance à venir
- BevZero Announces Strategic Partnership with TOMSA Desil to Distribute equipment for sustainability in the wine industry, as well as the development of Next-Gen Dealcoholization technology
- Editor's pick archive....

