
AstraZeneca: two trials show long-term efficacy and tolerability of Calquence (acalabrutinib) in chronic lymphocytic leukaemia
- ACE-CL-001 trial showed an overall response rate of 97% with a sustained safety profile for previously untreated patients after more than four years
- In pivotal ASCEND trial, 82% of patients with relapsed or refractory disease treated with Calquence remained progression free at 18 months vs. 48% for comparators
(PRESS RELEASE) CAMBRIDGE, 12-Jun-2020 — /EuropaWire/ — British-Swedish multinational pharmaceutical and biopharmaceutical company AstraZeneca has announced that its Calquence (acalabrutinib), which is a next-generation, selective inhibitor of Bruton’s tyrosine kinase (BTK), has showed long-term efficacy and tolerability for patients with chronic lymphocytic leukaemia, one of the most common types of adult leukaemia.¹,²,³ Detailed results from both the Phase II ACE-CL-001 trial and the pivotal Phase III ASCEND trial showed the long-term efficacy and tolerability of Calquence (acalabrutinib) in chronic lymphocytic leukaemia (CLL) and they will be presented during the Virtual Edition of the 25th European Hematology Association (EHA) Annual Congress, 11 to 14 June 2020.
In the single-arm ACE-CL-001 trial, 86% of CLL patients treated with Calquence as a 1st-line monotherapy remained on treatment at a median follow up of more than four years. The trial showed an overall response rate of 97% (7% complete response; 90% partial response) and a 100% overall response rate in subgroups of patients with high-risk disease characteristics, including genomic aberrations (17p deletion and TP53 mutation), immunoglobulin mutation status (unmutated IGHV), and complex karyotype. Safety findings showed no new long-term issues.1,4
In the final analysis of ASCEND, an estimated 82% of patients with relapsed or refractory CLL treated with Calquence remained alive and free from disease progression at 18 months compared with 48% of patients on rituximab combined with idelalisib or bendamustine.2 The trial previously met the primary endpoint of Independent Review Committee-assessed progression-free survival at the interim analysis.5
Richard R. Furman, Director of the CLL Research Center, Weill Cornell Medicine said: “These data demonstrate no new safety concerns for acalabrutinib, confirming its ability to safely provide meaningful, long-term clinical benefit for patients with treatment-naive and relapsed or refractory disease. The safety profile of acalabrutinib makes treatment to progression an important and plausible option for patients.”
José Baselga, Executive Vice President, Oncology R&D said: “These long-term data reaffirm that Calquence delivers a durable response with a favourable safety profile for chronic lymphocytic leukaemia patients. Patients with chronic lymphocytic leukaemia are typically 70 years or older with comorbidities and often require treatment over a long time, making the sustained safety and efficacy profile highly relevant to their quality of life.”
Results from the Phase II ACE-CL-001 trial informed the development of the pivotal Phase III ELEVATE TN trial, which, along with findings from the Phase III ASCEND trial, formed the basis for the US approval of Calquence for the treatment of patients with CLL or small lymphocytic lymphoma (SLL).
Calquence in previously untreated CLL: 4.4-year follow-up from Phase II trial (abstract #S163)
The Phase II ACE-CL-001 trial investigated safety and efficacy of Calquence (100mg twice-daily [n=62] or 200mg once-daily [n=37]) in previously untreated patients with CLL.1 On 1 May 2015, patients receiving the 200mg dosing regimen were switched to the 100mg regimen.1
Key data from the Calquence Phase II ACE-CL-001 trial1
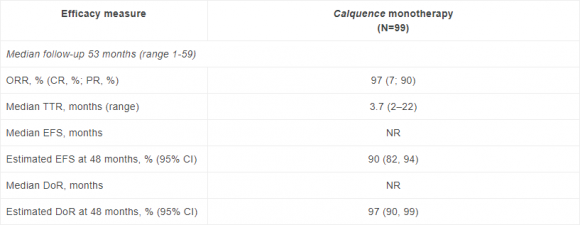
CI, confidence interval; CR, complete response; DoR, duration of response; EFS, event free survival; TTR, time to response; NR, not reached; ORR, overall response rate; PR, partial response
Response rates were 100% in each subgroup of patients with high-risk disease characteristics (unmutated IGHV [n=57], 17p deletion [n=9], TP53 mutation [n=9], and complex karyotype [n=12]), and reduction in lymph node disease was noted in all patients tested (n=97).1
At the time of data cut-off, 85 (86%) patients receiving Calquence remained on treatment. Six patients discontinued treatment due to adverse events (AEs) and three patients discontinued for progressive disease (PD). No patient discontinued Calquence due to bleeding events, hypertension, or atrial fibrillation. Incidence of AEs generally diminished with time on the trial. The most common AEs (≥40%) of any grade in the trial were diarrhoea (52%), headache (45%), upper respiratory tract infection (44%), arthralgia (42%), and contusion (42%). All-grade and Grade ≥3 events of clinical interest included infection (84% and 15%, respectively), bleeding events (66%, 3%), hypertension (22%, 11%), leukopenia (9%, 9%), and thrombocytopenia (3%, 1%). Atrial fibrillation (all grades) occurred in 5% of patients with Grade ≥3 occurring in 2%. Second primary malignancies (SPM) excluding non-melanoma skin (all grades) occurred in 11% of patients.1 Serious adverse events (SAEs) were reported in 38% of patients. SAEs occurring in more than two patients included pneumonia (n=4) and sepsis (n=3).1
Final results of Calquence Phase III ASCEND trial in relapsed or refractory CLL (abstract #S159)
ASCEND was a global, randomised, multicentre, open-label, Phase III trial that investigated the efficacy and safety of Calquence (100mg twice-daily) versus investigator’s choice of rituximab combined with idelalisib (IdR) or bendamustine (BR) in patients with relapsed or refractory CLL.2
Key data from the final analysis of the Calquence Phase III ASCEND trial2
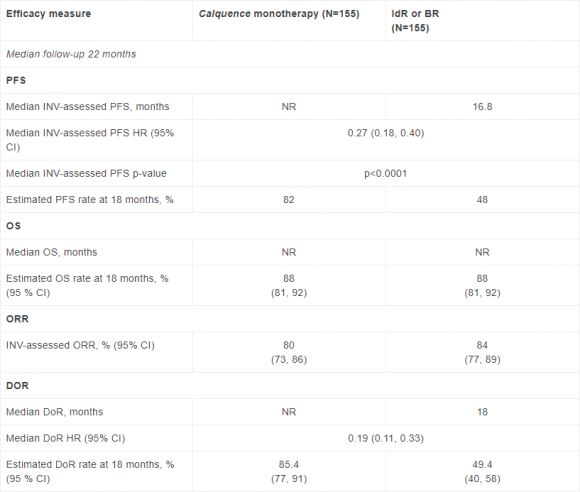
BR, rituximab in combination with bendamustine; CI, confidence interval, DoR, duration of response; HR, hazard ratio; IdR, rituximab in combination with idelalisib; INV, investigator; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival
Sixteen per cent of patients on Calquence, 56% of patients on IdR, and 17% of patients on BR discontinued treatment because of AEs. Common AEs occurring in greater than 15% of patients of any grade in the Calquence arm of the trial included headache (22%), neutropenia (21%), diarrhoea (20%), upper respiratory tract infection (20%), cough (16%), and anaemia (16%). Events of clinical interest for Calquence versus controls included atrial fibrillation (all grade, 6% and 3%, respectively), major haemorrhage (all grade, 3% in both arms), infections (Grade ≥3, 20% and 25%, respectively), and SPM excluding non-melanoma skin cancer (all grade, 5% and 2%, respectively). SAEs (any grade) occurred in 33% of patients receiving Calquence, 56% of IdR patients, and 26% of BR patients.2
Chronic lymphocytic leukaemia
Chronic lymphocytic leukaemia (CLL) is one of the most common types of leukaemia in adults, with an estimated 105,000 new cases globally in 2016 and 21,040 new cases in the US in 2020, and the number of people living with CLL is expected to grow with improved treatment as patients live longer with the disease.3,6,7,8 In CLL, too many blood stem cells in the bone marrow become abnormal lymphocytes and these abnormal cells have difficulty fighting infections.3 As the number of abnormal cells grows there is less room for healthy white blood cells, red blood cells, and platelets.3 This could result in anaemia, infection, and bleeding.3 B-cell receptor signalling through Bruton’s tyrosine kinase is one of the essential growth pathways for CLL.
Calquence
Calquence (acalabrutinib) is a next-generation, selective inhibitor of Bruton’s tyrosine kinase (BTK). Calquence binds covalently to BTK, thereby inhibiting its activity.4,9 In B-cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion.4
Calquence is approved for the treatment of adult patients with chronic lymphocytic leukaemia (CLL) in nine countries and for adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy in 14 countries. The US MCL indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. As part of an extensive clinical development programme, AstraZeneca and Acerta Pharma are currently evaluating Calquence in 23 company-sponsored clinical trials. Calquence is being developed for the treatment of multiple B-cell blood cancers including CLL, MCL, diffuse large B-cell lymphoma, Waldenström macroglobulinaemia, follicular lymphoma, and other haematologic malignancies.
AstraZeneca in haematology
Leveraging its strength in oncology, AstraZeneca has established haematology as one of four key oncology disease areas of focus. The Company’s haematology franchise includes two US FDA-approved medicines and a robust global development programme for a broad portfolio of potential blood cancer treatments. Acerta Pharma serves as AstraZeneca’s haematology research and development arm. AstraZeneca partners with like-minded science-led companies to advance the discovery and development of therapies to address unmet need.
AstraZeneca in oncology
AstraZeneca has a deep-rooted heritage in oncology and offers a quickly growing portfolio of new medicines that has the potential to transform patients’ lives and the Company’s future. With six new medicines launched between 2014 and 2020, and a broad pipeline of small molecules and biologics in development, the Company is committed to advance oncology as a key growth driver for AstraZeneca focused on lung, ovarian, breast and blood cancers. In addition to AstraZeneca’s main capabilities, the Company is actively pursuing innovative partnerships and investments that accelerate the delivery of our strategy, as illustrated by the investment in Acerta Pharma in haematology.
By harnessing the power of four scientific platforms – Immuno-Oncology, Tumour Drivers and Resistance, DNA Damage Response and Antibody Drug Conjugates – and by championing the development of personalised combinations, AstraZeneca has the vision to redefine cancer treatment and one day eliminate cancer as a cause of death.
AstraZeneca
AstraZeneca (LSE/STO/NYSE: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of diseases in three therapy areas – Oncology, Cardiovascular, Renal and Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Media
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
References
1. Byrd JC, et al. Acalabrutinib in Treatment-Naïve Chronic Lymphocytic Leukemia: Mature Results From Phase 2 Study Demonstrating Durable Remissions and Long-Term Tolerability. Abstract S163 presented at the Virtual Edition of the 15th European Hematology Association (EHA) Annual Meeting. Available online. Accessed June 2020.
2. Ghia P, et al. Acalabrutinib (Acala) vs Idelalisib plus Rituximab (IdR) or Bendamustine plus Rituximab (BR) in Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): ASCEND Final Results. Abstract S159 presented at the Virtual Edition of the 15th European Hematology Association (EHA) Annual Meeting. Available online. Accessed June 2020.
3. National Cancer Institute. Chronic Lymphocytic Leukemia Treatment (PDQ®)–Patient Version. Available online. Accessed June 2020.
4. Calquence (acalabrutinib) [prescribing information]. Wilmington, DE; AstraZeneca Pharmaceuticals LP; 2019.
5. Ghia P, et al. ASCEND Phase 3 Study of Acalabrutinib vs Investigator’s Choice of Rituximab Plus Idelalisib (IdR) or Bendamustine (BR) in Patients with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL). Abstract LB2606 at the 2019 European Hematology Association (EHA) Annual Meeting. Available online. Accessed June 2020.
6. Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016. JAMA Oncol. 2018;4(11):1553-1568.
7. American Cancer Society. Key Statistics for Chronic Lymphocytic Leukemia. Available online. Accessed June 2020.
8. Jain N, et al. Prevalence and Economic Burden of Chronic Lymphocytic Leukemia (CLL) in the Era of Oral Targeted Therapies. Blood. 2015;126:871.
9. Wu J, Zhang M & Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9(21).
SOURCE: AstraZeneca
- BREAKING NEWS: New Podcast “Spreading the Good BUZZ” Hosted by Josh and Heidi Case Launches July 7th with Explosive Global Reach and a Mission to Transform Lives Through Hope and Community in Recovery
- Cha Cha Cha kohtub krüptomaailmaga: Winz.io teeb koostööd Euroopa visionääri ja staari Käärijäga
- Digi Communications N.V. announces Conditional stock options granted to Executive Directors of the Company, for the year 2025, based on the general shareholders’ meeting approval from 25 June 20244
- Cha Cha Cha meets crypto: Winz.io partners with European visionary star Käärijä
- Digi Communications N.V. announces the exercise of conditional share options by the executive directors of the Company, for the year 2024, as approved by the Company’s OGSM from 25 June 2024
- “Su Fortuna Se Ha Construido A Base de La Defraudación Fiscal”: Críticas Resurgen Contra Ricardo Salinas en Medio de Nuevas Revelaciones Judiciales y Fiscaleso
- Digi Communications N.V. announces the availability of the instruction regarding the payment of share dividend for the 2024 financial year
- SOILRES project launches to revive Europe’s soils and future-proof farming
- Josh Case, ancien cadre d’ENGIE Amérique du Nord, PDG de Photosol US Renewable Energy et consultant d’EDF Amérique du Nord, engage aujourd’hui toute son énergie dans la lutte contre la dépendance
- Bizzy startet den AI Sales Agent in Deutschland: ein intelligenter Agent zur Automatisierung der Vertriebspipeline
- Bizzy lance son agent commercial en France : un assistant intelligent qui automatise la prospection
- Bizzy lancia l’AI Sales Agent in Italia: un agente intelligente che automatizza la pipeline di vendita
- Bizzy lanceert AI Sales Agent in Nederland: slimme assistent automatiseert de sales pipeline
- Bizzy startet AI Sales Agent in Österreich: ein smarter Agent, der die Sales-Pipeline automatisiert
- Bizzy wprowadza AI Sales Agent w Polsce: inteligentny agent, który automatyzuje budowę lejka sprzedaży
- Bizzy lanza su AI Sales Agent en España: un agente inteligente que automatiza la generación del pipeline de ventas
- Bizzy launches AI Sales Agent in the UK: a smart assistant that automates sales pipeline generation
- As Sober.Buzz Community Explodes Its Growth Globally it is Announcing “Spreading the Good BUZZ” Podcast Hosted by Josh Case Debuting July 7th
- Digi Communications N.V. announces the OGMS resolutions and the availability of the approved 2024 Annual Report
- Escándalo Judicial en Aumento Alarma a la Opinión Pública: Suprema Corte de México Enfrenta Acusaciones de Favoritismo hacia el Aspirante a Magnate Ricardo Salinas Pliego
- Winz.io Named AskGamblers’ Best Casino 2025
- Kissflow Doubles Down on Germany as a Strategic Growth Market with New AI Features and Enterprise Focus
- Digi Communications N.V. announces Share transaction made by a Non-Executive Director of the Company with class B shares
- Salinas Pliego Intenta Frenar Investigaciones Financieras: UIF y Expertos en Corrupción Prenden Alarmas
- Digital integrity at risk: EU Initiative to strengthen the Right to be forgotten gains momentum
- Orden Propuesta De Arresto E Incautación Contra Ricardo Salinas En Corte De EE.UU
- Digi Communications N.V. announced that Serghei Bulgac, CEO and Executive Director, sold 15,000 class B shares of the company’s stock
- PFMcrypto lancia un sistema di ottimizzazione del reddito basato sull’intelligenza artificiale: il mining di Bitcoin non è mai stato così facile
- Azteca Comunicaciones en Quiebra en Colombia: ¿Un Presagio para Banco Azteca?
- OptiSigns anuncia su expansión Europea
- OptiSigns annonce son expansion européenne
- OptiSigns kündigt europäische Expansion an
- OptiSigns Announces European Expansion
- Digi Communications NV announces release of Q1 2025 financial report
- Banco Azteca y Ricardo Salinas Pliego: Nuevas Revelaciones Aumentan la Preocupación por Riesgos Legales y Financieros
- Digi Communications NV announces Investors Call for the presentation of the Q1 2025 Financial Results
- Digi Communications N.V. announces the publication of the 2024 Annual Financial Report and convocation of the Company’s general shareholders meeting for June 18, 2025, for the approval of, among others, the 2024 Annual Financial Report, available on the Company’s website
- La Suprema Corte Sanciona a Ricardo Salinas de Grupo Elektra por Obstrucción Legal
- Digi Communications N.V. announces the conclusion of an Incremental to the Senior Facilities Agreement dated 21 April 2023
- 5P Europe Foundation: New Initiative for African Children
- 28-Mar-2025: Digi Communications N.V. announces the conclusion of Facilities Agreements by companies within Digi Group
- Aeroluxe Expeditions Enters U.S. Market with High-Touch Private Jet Journeys—At a More Accessible Price ↗️
- SABIO GROUP TAKES IT’S ‘DISRUPT’ CX PROGRAMME ACROSS EUROPE
- EU must invest in high-quality journalism and fact-checking tools to stop disinformation
- ¿Está Banco Azteca al borde de la quiebra o de una intervención gubernamental? Preocupaciones crecientes sobre la inestabilidad financiera
- Netmore and Zenze Partner to Deploy LoRaWAN® Networks for Cargo and Asset Monitoring at Ports and Terminals Worldwide
- Rise Point Capital: Co-investing with Independent Sponsors to Unlock International Investment Opportunities
- Netmore Launches Metering-as-a-Service to Accelerate Smart Metering for Water and Gas Utilities
- Digi Communications N.V. announces that a share transaction was made by a Non-Executive Director of the Company with class B shares
- La Ballata del Trasimeno: Il Mediometraggio si Trasforma in Mini Serie
- Digi Communications NV Announces Availability of 2024 Preliminary Financial Report
- Digi Communications N.V. announces the recent evolution and performance of the Company’s subsidiary in Spain
- BevZero Equipment Sales and Distribution Enhances Dealcoholization Capabilities with New ClearAlc 300 l/h Demonstration Unit in Spain Facility
- Digi Communications NV announces Investors Call for the presentation of the 2024 Preliminary Financial Results
- Reuters webinar: Omnibus regulation Reuters post-analysis
- Patients as Partners® Europe Launches the 9th Annual Event with 2025 Keynotes, Featured Speakers and Topics
- eVTOLUTION: Pioneering the Future of Urban Air Mobility
- Reuters webinar: Effective Sustainability Data Governance
- Las acusaciones de fraude contra Ricardo Salinas no son nuevas: una perspectiva histórica sobre los problemas legales del multimillonario
- Digi Communications N.V. Announces the release of the Financial Calendar for 2025
- USA Court Lambasts Ricardo Salinas Pliego For Contempt Of Court Order
- 3D Electronics: A New Frontier of Product Differentiation, Thinks IDTechEx
- Ringier Axel Springer Polska Faces Lawsuit for Over PLN 54 million
- Digi Communications N.V. announces the availability of the report on corporate income tax information for the financial year ending December 31, 2023
- Unlocking the Multi-Million-Dollar Opportunities in Quantum Computing
- Digi Communications N.V. Announces the Conclusion of Facilities Agreements by Companies within Digi Group
- The Hidden Gem of Deep Plane Facelifts
- KAZANU: Redefining Naturist Hospitality in Saint Martin ↗️
- New IDTechEx Report Predicts Regulatory Shifts Will Transform the Electric Light Commercial Vehicle Market
- Almost 1 in 4 Planes Sold in 2045 to be Battery Electric, Finds IDTechEx Sustainable Aviation Market Report
- Digi Communications N.V. announces the release of Q3 2024 financial results
- Digi Communications NV announces Investors Call for the presentation of the Q3 2024 Financial Results
- Pilot and Electriq Global announce collaboration to explore deployment of proprietary hydrogen transport, storage and power generation technology
- Digi Communications N.V. announces the conclusion of a Memorandum of Understanding by its subsidiary in Romania
- Digi Communications N.V. announces that the Company’s Portuguese subsidiary finalised the transaction with LORCA JVCO Limited
- Digi Communications N.V. announces that the Portuguese Competition Authority has granted clearance for the share purchase agreement concluded by the Company’s subsidiary in Portugal
- OMRON Healthcare introduceert nieuwe bloeddrukmeters met AI-aangedreven AFib-detectietechnologie; lancering in Europa september 2024
- OMRON Healthcare dévoile de nouveaux tensiomètres dotés d’une technologie de détection de la fibrillation auriculaire alimentée par l’IA, lancés en Europe en septembre 2024
- OMRON Healthcare presenta i nuovi misuratori della pressione sanguigna con tecnologia di rilevamento della fibrillazione atriale (AFib) basata sull’IA, in arrivo in Europa a settembre 2024
- OMRON Healthcare presenta los nuevos tensiómetros con tecnología de detección de fibrilación auricular (FA) e inteligencia artificial (IA), que se lanzarán en Europa en septiembre de 2024
- Alegerile din Moldova din 2024: O Bătălie pentru Democrație Împotriva Dezinformării
- Northcrest Developments launches design competition to reimagine 2-km former airport Runway into a vibrant pedestrianized corridor, shaping a new era of placemaking on an international scale
- The Road to Sustainable Electric Motors for EVs: IDTechEx Analyzes Key Factors
- Infrared Technology Breakthroughs Paving the Way for a US$500 Million Market, Says IDTechEx Report
- MegaFair Revolutionizes the iGaming Industry with Skill-Based Games
- European Commission Evaluates Poland’s Media Adherence to the Right to be Forgotten
- Global Race for Autonomous Trucks: Europe a Critical Region Transport Transformation
- Digi Communications N.V. confirms the full redemption of €450,000,000 Senior Secured Notes
- AT&T Obtiene Sentencia Contra Grupo Salinas Telecom, Propiedad de Ricardo Salinas, Sus Abogados se Retiran Mientras Él Mueve Activos Fuera de EE.UU. para Evitar Pagar la Sentencia
- Global Outlook for the Challenging Autonomous Bus and Roboshuttle Markets
- Evolving Brain-Computer Interface Market More Than Just Elon Musk’s Neuralink, Reports IDTechEx
- Latin Trails Wraps Up a Successful 3rd Quarter with Prestigious LATA Sustainability Award and Expands Conservation Initiatives ↗️
- Astor Asset Management 3 Ltd leitet Untersuchung für potenzielle Sammelklage gegen Ricardo Benjamín Salinas Pliego von Grupo ELEKTRA wegen Marktmanipulation und Wertpapierbetrug ein
- Digi Communications N.V. announces that the Company’s Romanian subsidiary exercised its right to redeem the Senior Secured Notes due in 2025 in principal amount of €450,000,000
- Astor Asset Management 3 Ltd Inicia Investigación de Demanda Colectiva Contra Ricardo Benjamín Salinas Pliego de Grupo ELEKTRA por Manipulación de Acciones y Fraude en Valores
- Astor Asset Management 3 Ltd Initiating Class Action Lawsuit Inquiry Against Ricardo Benjamín Salinas Pliego of Grupo ELEKTRA for Stock Manipulation & Securities Fraud
- Digi Communications N.V. announced that its Spanish subsidiary, Digi Spain Telecom S.L.U., has completed the first stage of selling a Fibre-to-the-Home (FTTH) network in 12 Spanish provinces
- Natural Cotton Color lancia la collezione "Calunga" a Milano
- Astor Asset Management 3 Ltd: Salinas Pliego Incumple Préstamo de $110 Millones USD y Viola Regulaciones Mexicanas
- Astor Asset Management 3 Ltd: Salinas Pliego Verstößt gegen Darlehensvertrag über 110 Mio. USD und Mexikanische Wertpapiergesetze
- ChargeEuropa zamyka rundę finansowania, której przewodził fundusz Shift4Good tym samym dokonując historycznej francuskiej inwestycji w polski sektor elektromobilności
- Strengthening EU Protections: Robert Szustkowski calls for safeguarding EU citizens’ rights to dignity
- Digi Communications NV announces the release of H1 2024 Financial Results
- Digi Communications N.V. announces that conditional stock options were granted to a director of the Company’s Romanian Subsidiary
- Digi Communications N.V. announces Investors Call for the presentation of the H1 2024 Financial Results
- Digi Communications N.V. announces the conclusion of a share purchase agreement by its subsidiary in Portugal
- Digi Communications N.V. Announces Rating Assigned by Fitch Ratings to Digi Communications N.V.
- Digi Communications N.V. announces significant agreements concluded by the Company’s subsidiaries in Spain
- SGW Global Appoints Telcomdis as the Official European Distributor for Motorola Nursery and Motorola Sound Products
- Digi Communications N.V. announces the availability of the instruction regarding the payment of share dividend for the 2023 financial year
- Digi Communications N.V. announces the exercise of conditional share options by the executive directors of the Company, for the year 2023, as approved by the Company’s Ordinary General Shareholders’ Meetings from 18th May 2021 and 28th December 2022
- Digi Communications N.V. announces the granting of conditional stock options to Executive Directors of the Company based on the general shareholders’ meeting approval from 25 June 2024
- Digi Communications N.V. announces the OGMS resolutions and the availability of the approved 2023 Annual Report
- Czech Composer Tatiana Mikova Presents Her String Quartet ‘In Modo Lidico’ at Carnegie Hall
- SWIFTT: A Copernicus-based forest management tool to map, mitigate, and prevent the main threats to EU forests
- WickedBet Unveils Exciting Euro 2024 Promotion with Boosted Odds
- Museum of Unrest: a new space for activism, art and design
- Digi Communications N.V. announces the conclusion of a Senior Facility Agreement by companies within Digi Group
- Digi Communications N.V. announces the agreements concluded by Digi Romania (formerly named RCS & RDS S.A.), the Romanian subsidiary of the Company
- Green Light for Henri Hotel, Restaurants and Shops in the “Alter Fischereihafen” (Old Fishing Port) in Cuxhaven, opening Summer 2026
- Digi Communications N.V. reports consolidated revenues and other income of EUR 447 million, adjusted EBITDA (excluding IFRS 16) of EUR 140 million for Q1 2024
- Digi Communications announces the conclusion of Facilities Agreements by companies from Digi Group
- Digi Communications N.V. Announces the convocation of the Company’s general shareholders meeting for 25 June 2024 for the approval of, among others, the 2023 Annual Report
- Digi Communications NV announces Investors Call for the presentation of the Q1 2024 Financial Results
- Digi Communications intends to propose to shareholders the distribution of dividends for the fiscal year 2023 at the upcoming General Meeting of Shareholders, which shall take place in June 2024
- Digi Communications N.V. announces the availability of the Romanian version of the 2023 Annual Report
- Digi Communications N.V. announces the availability of the 2023 Annual Report
- International Airlines Group adopts Airline Economics by Skailark ↗️
- BevZero Spain Enhances Sustainability Efforts with Installation of Solar Panels at Production Facility
- Digi Communications N.V. announces share transaction made by an Executive Director of the Company with class B shares
- BevZero South Africa Achieves FSSC 22000 Food Safety Certification
- Digi Communications N.V.: Digi Spain Enters Agreement to Sell FTTH Network to International Investors for Up to EUR 750 Million
- Patients as Partners® Europe Announces the Launch of 8th Annual Meeting with 2024 Keynotes and Topics
- driveMybox continues its international expansion: Hungary as a new strategic location
- Monesave introduces Socialised budgeting: Meet the app quietly revolutionising how users budget
- Digi Communications NV announces the release of the 2023 Preliminary Financial Results
- Digi Communications NV announces Investors Call for the presentation of the 2023 Preliminary Financial Results
- Lensa, един от най-ценените търговци на оптика в Румъния, пристига в България. Първият шоурум е открит в София
- Criando o futuro: desenvolvimento da AENO no mercado de consumo em Portugal
- Digi Communications N.V. Announces the release of the Financial Calendar for 2024
- Customer Data Platform Industry Attracts New Participants: CDP Institute Report
- eCarsTrade annonce Dirk Van Roost au poste de Directeur Administratif et Financier: une décision stratégique pour la croissance à venir
- BevZero Announces Strategic Partnership with TOMSA Desil to Distribute equipment for sustainability in the wine industry, as well as the development of Next-Gen Dealcoholization technology
- Editor's pick archive....

